Technology Platform
Our technology harnesses the body’s natural ‘self-recognition’ system to control aberrant inflammation. By targeting the interaction between sialic acids on cell surfaces and Siglec immune receptors, we aim to modulate immune responses and open new possibilities for treating chronic disease.
Sialic Acid Background
Sialic acids are present on the surface of all living human cells. The typical human cell displays tens of millions of sialic acid molecules. Together with other sugars and proteins, this outer layer of the cell is called the “glycocalyx”. Sialic Acids play pivotal roles in cellular communication and function, serving as a receptor for various biological signaling pathways. They are an important component of the body's natural “self” recognition and a control “checkpoint” for chronic inflammation and disease. Macrophages, microglia, and monocytes, as well as complement factor H all recognize sialic acid as “self” and this recognition helps to downregulate inflammatory cells as well as the complement pathway on all surfaces.
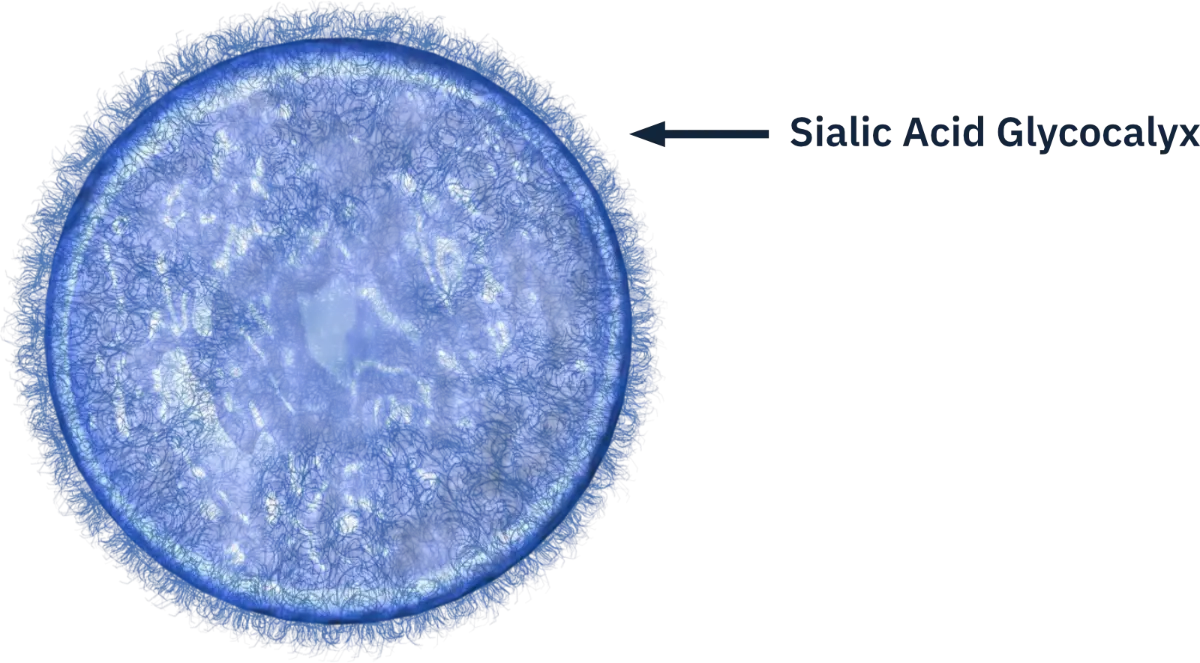
Siglec Biology
Siglec receptors are immune checkpoint receptors that modulate the response of immune cells. Siglecs (Sialic acid binding immunoglobulin-like lectins) discriminate between “self” and “non-self” to modulate the immune response by specifically binding to sialic acids.
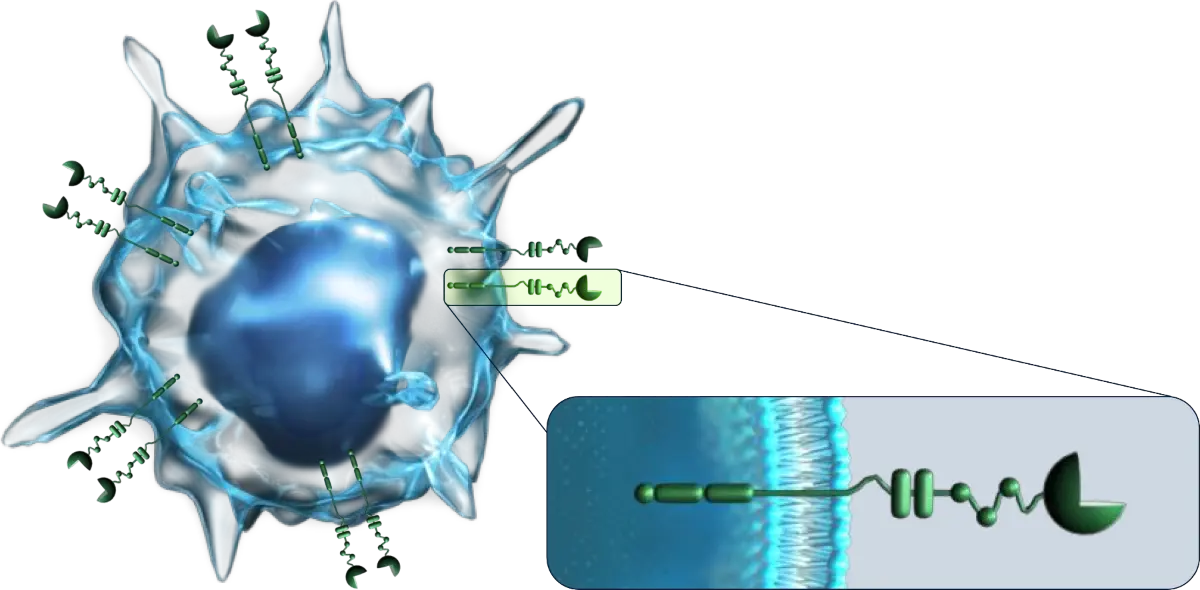
Many Siglec Receptors are Inhibitory and are the Main Receptors that Differentiate "Self" from "Non-Self".
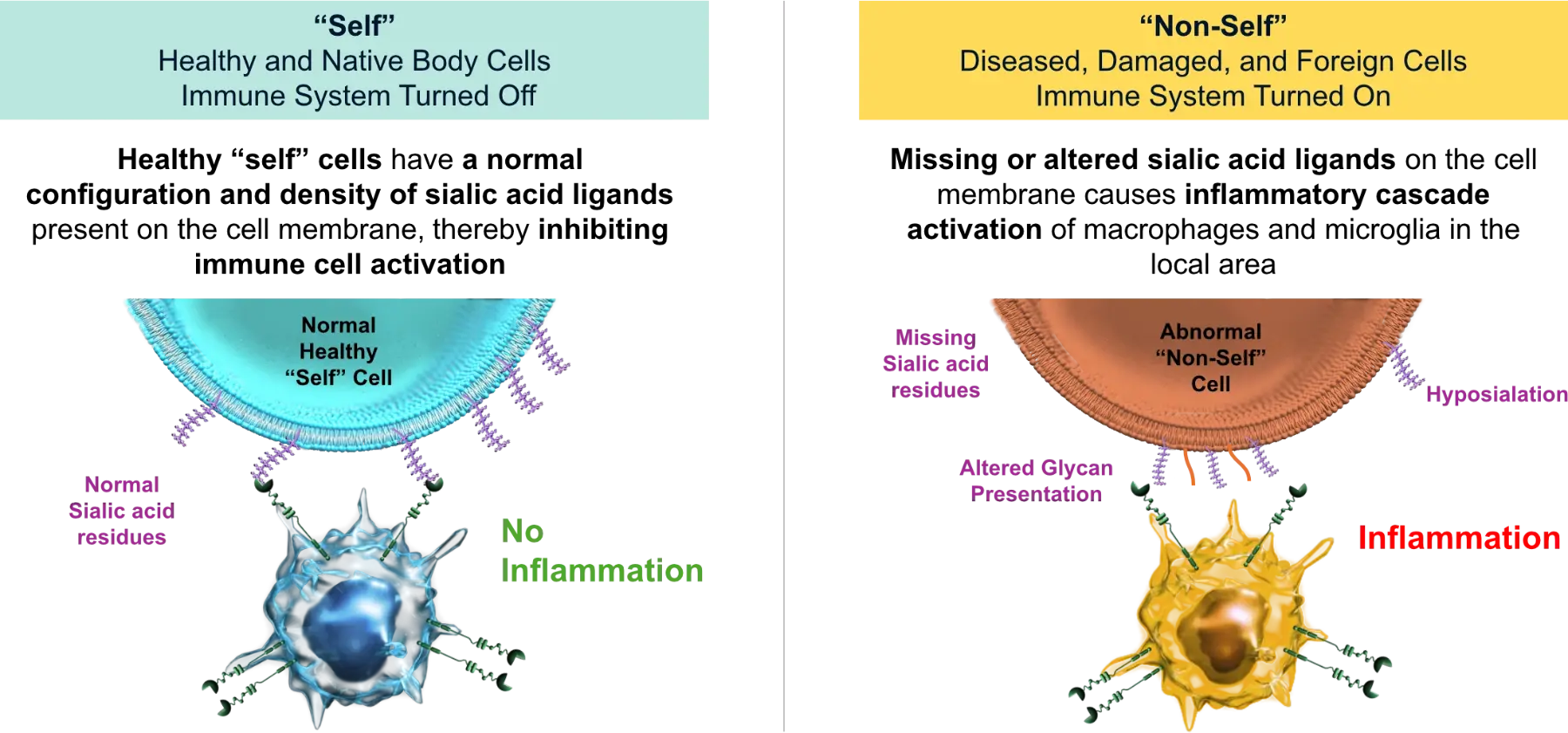
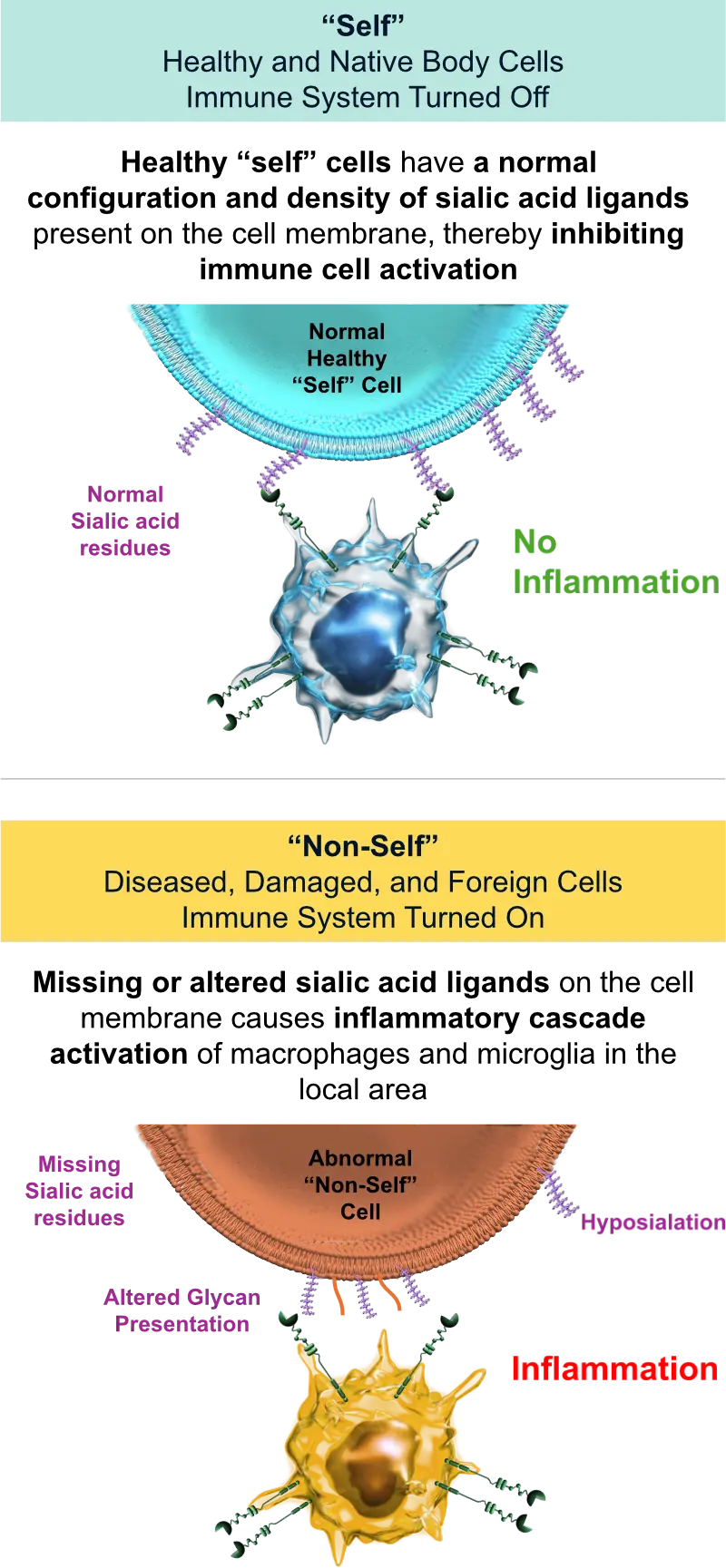
Our lead asset, AVD-104 targets this axis to treat AMD.
Explore AVD-104